Our latest launches that bring innovative solutions to patients across the world

The first immunotherapy approved by major global regulators for recurrent or metastatic nasopharyngeal carcinoma (RM-NPC). In India, it introduces immuno-oncology as a first-line treatment for RM-NPC in combination with chemotherapy.

Our first biosimilar approved and launched in the UK. It is a treatment for several types of cancer, including colorectal, lung, brain, kidney, cervical, and ovarian cancers. Bevacizumab works by blocking a protein called VEGF, slowing the growth and spread of cancer.

A long-acting, USFDA-approved treatment for HIV-1 infection. Our work with Lenacapavir supports our goal to expand HIV treatment and prevention, especially in high-burden regions. We aim to manufacture and commercialise Lenacapavir across 120 countries, in partnership with Gilead Sciences.
We operate across four main segments: Generics (branded, unbranded, and biosimilars), API & Services (development, manufacturing, and research support), Innovative Medicines, and Consumer Health solutions. We are also expanding our portfolio across therapies through in-licensing and partnering with innovative companies across the globe.


A patient support program where gastroenterologists help people with ulcerative colitis better understand and manage Inflammatory Bowel Disease through personalised guidance on diet, exercise, and stress.

An initiative in Germany providing useful health information to patients. It also offers certified online courses to improve patient care, and help them navigate treatment and the healthcare system.

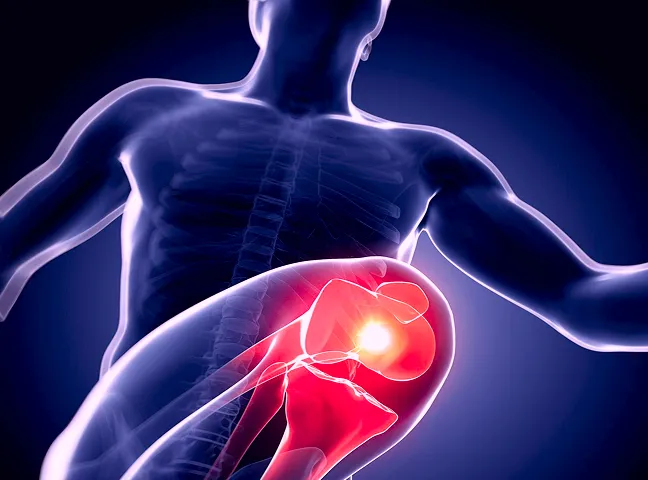
Sessions developed for individuals with osteoarthritis, designed to guide them in understanding effective joint care. These doctor-led sessions help patients learn about the right exercises, healthy habits and ways to manage pain, and stay active.
"Our mission is to turn
scientific possibilities into real hope,
addressing urgent patient needs
and helping improve lives."

Countries
Active US Abbreviated New Drug Applications (ANDAs)
Active US Drug Master Files (DMFs)
Patients Reached (FY'25)
Active EU Dossiers Filed
Active EU Drug Master Files (DMFs)
Helping bring effective treatments to patients faster and at a lower cost to ease the burden of disease.
Skorolox is the first loxoprofen INN available in Russia. It is used to relieve symptoms of acute upper respiratory tract infections, and to treat musculoskeletal and postoperative pain.
A novel drug launched in India to protect newborns and infants from lung infections caused by Respiratory Syncytial Virus in the first season of exposure.
A USFDA-approved option for Chronic Idiopathic Constipation in adults, which acts on gut receptors to help support normal digestive function.

"Our partners trust us because
we have a long standing reputation
for doing the right thing, no matter what.
We are committed to accelerating
access to 1.5bn patients and it can
only be done by working with innovative
companies doing great work across the globe."
We work together with our partners to bring healthcare solutions across communities

Working to develop an injectable contraceptive for low and middle income countries, advancing women's health, improving family planning options, and reducing health inequities.
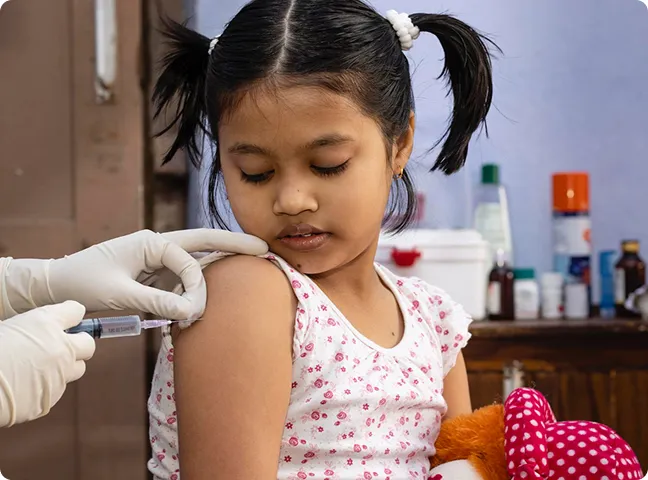
Partnering to distribute pediatric and adult vaccines in India, expanding immunization coverage, and strengthening disease prevention.
Collaborating to expand access to essential cancer therapies approved for more than 40 types of cancer, strengthening oncology care and supporting patients with high-burden diseases worldwide.
Our teams are solving complex challenges with science and technology, keeping patients at the core
"Complex injectables like peptides are redefining patient care, and we’re at the forefront of that transformation."

"In the next 10-20 years the field of cell and gene therapy is going to evolve significantly."

"We have a well-developed research pipeline in India, with a primary focus on cancer treatments."
"Quality is pervasive.
It's in how we work,
how we think, and in the
culture of doing things right.
When that mindset is shared,
everyone becomes responsible for quality."
"Quality is pervasive.
It's in how we work,
how we think, and in the
culture of doing things right.
When that mindset is shared,
everyone becomes responsible for quality."
We continue working towards reducing our environmental footprint, addressing ecological risks from our operations, and are progressing towards carbon neutrality

By implementing initiatives such as wastewater recovery, grey water use, and more, we reduced freshwater consumption by ~70,000 KL from April to September 2023.

By conducting site-level climate risk assessments and improving supplier engagement by sustainable procurement, we identified key vulnerabilities and reduced Scope 3 emissions intensity by 31% since FY2023.

By implementing cost improvement projects and strengthening site-level waste checks, we achieved 20-22% reduction in waste across 9 high-volume products.

By replacing fossil fuels with biofuels in boilers and expanding solar, wind, and hybrid energy use, we reduced Scope 1 and 2 emissions by 33% vs FY2023. Energy-saving initiatives also helped avoid 8,073 tonnes of CO₂ and saved 7.75 million kWh in FY2025.
Discover the science behind our products and how we work towards making a difference in patients' lives